PROJECT DETAILS
- Team Leader Dr Amos Folarin
- Contact Person Yatharth Ranjan, Malik Althobiani
- Website RADAR-base
- LinkedIn Amos Folarin
- Twitter @amosfolarin
Description
~~PAGE UNDER CONSTRUCTION~~
1.1 BACKGROUND
During these unprecedented times, an urgent measure must be taken to ensure vulnerable patients with diseases, like COPD and ILD, continue to receive the quality of care they need. Currently, Covid-19 is a challenge for anyone, but especially for such vulnerable patients with pre-existing conditions and diseases when their routine care cannot be done properly. Remote monitoring of physiology and symptoms of patients via wearable devices is more timely and essential than ever. It could be used to support these patients and detect any potential disease exacerbation or progression without putting them in situations where the risk of exposure to Covid-19 has more serious consequences.
1.2 Remote Monitoring
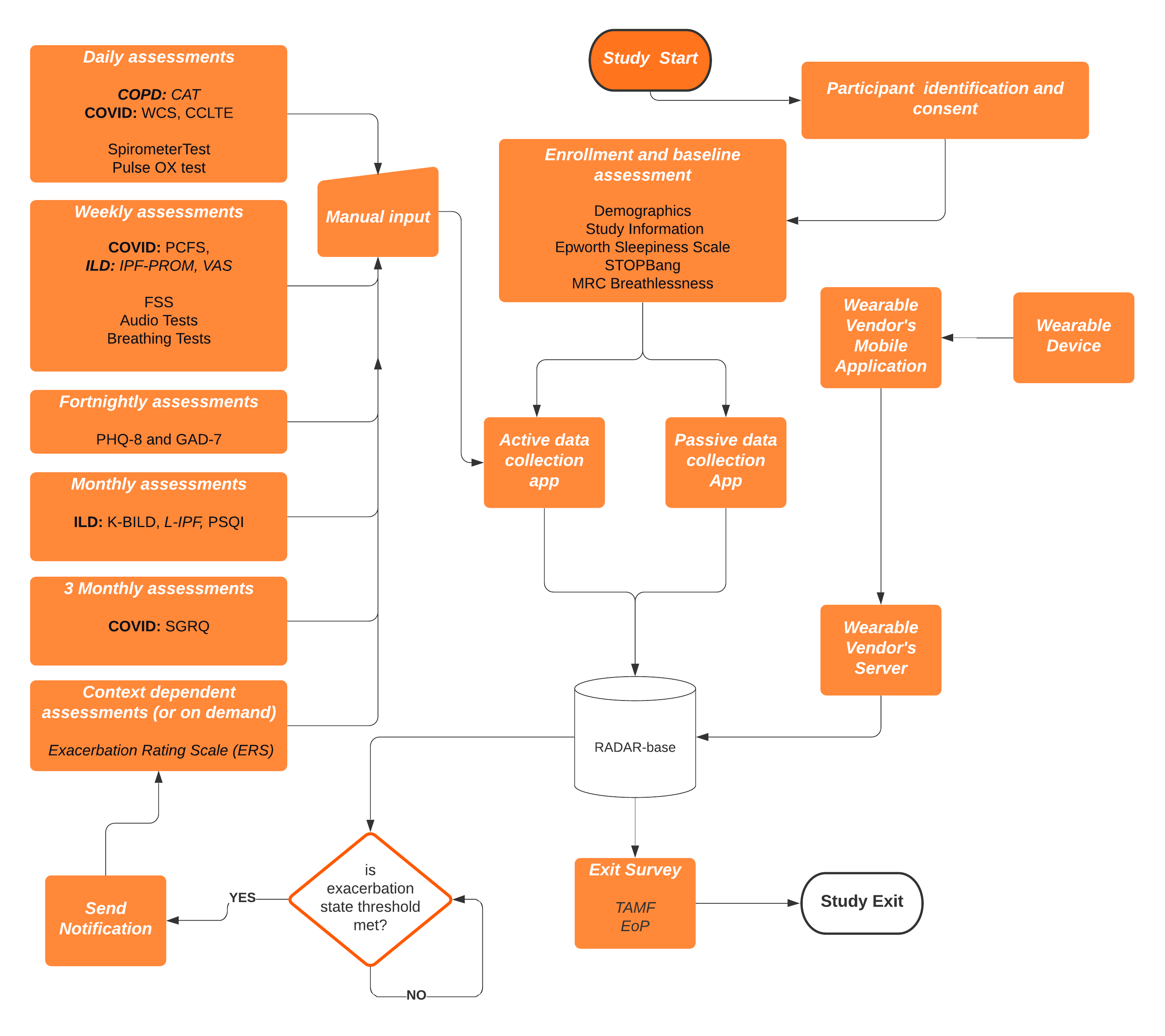
This study aims to use the open source RADAR-base mHealth platform to collect and analyse multiple datasets associated with respiratory disorders. This will include continuous data collected from wearable devices (e.g. heart rate, spO2, StO2), including pulse oximeters, spirometer, mobile phones (audio, location), digital tests and smartphone symptoms questionnaires.
The RADAR-base community emerged from the IMI project RADAR-CNS, where a consortium of clinicians, developers, researchers, patient organizations and EFPIA partners joined forces to explore the potential use of sensor data from wearable devices like fitness trackers and smartphones in research and healthcare. The RADAR-base platform is a scalable and inter-operable mHealth platform which provides capabilities for remote monitoring using passively (e.g. sensor data, wearables, IoT) and actively (e.g. questionnaires, digital tests). The platform developed at King’s College London is already being used in a number of large-scale longitudinal mental and physical health-related disorder projects (see https://radar-base.org)(1). The complete RADAR-base technology stack is available under an Apache 2 open source license and is supported by an active community of developers, researchers and clinicians who focus on continuously improving data quality, user experience, validation and extending the platform with new features and data sources.
RADAR-base also provides the potential to respond or alert in near-real time based on some state of the data being collected; this could include identifying e.g. an exacerbation and triggering a response, such as an intervention or follow-up questionnaires/tests or confirmation.
This pilot will help answer how remote monitoring may be used for lung disease patients who in many cases due to the pandemic may be shielding and offers additional benefits including participation without additional risk of travel or interaction with hospital staff.
1.3.1 Interstitial Lung Disease (ILD)
ILD, or lung fibrosis, is one of a spectrum of fibrotic diseases, associated with ageing, obesity, diabetes and pollution, that are responsible for ~45% of premature deaths in Western Europe. Of >90,000 patients in the UK with ILD, ~30,000 have idiopathic pulmonary fibrosis, IPF, the most severe form. IPF is a disease of unknown aetiology that is more frequent in males presenting mainly in the sixth and seventh decades of life. There is no cure and median survival, just 3-5 years following diagnosis is worse than for many cancers. As the fibrosis progresses this leads to impaired pulmonary function, respiratory failure and ultimately death. Throughout its course, IPF has significant effects on physical (dyspnoea, dry cough, weight loss and fatigue) and social (recreational activities, relationships) function, with severe consequences for the patient’s health related quality of life (HRQoL). Clinical courses are punctuated by episodes of worsening disease that may result in death. These acute exacerbations (AE-IPF) are estimated to occur in 4-20% of patients each year but the true incidence and impact is not known.
Management of AE-IPF involves establishing the diagnosis and excluding other causes of increasing dyspnea, excluding infection and considering the use of steroids, antibiotics and/ or anticoagulation- none of which has been shown to be of benefit. The trajectory of patients with IPF is heterogeneous with great variability in disease course, some progress slowly whereas others progress more rapidly, and this can cause emotional distress and anxiety. Patient Reported Outcome Measures (PROMs) are used to measure HRQoL, assess symptoms and evaluate disease progression. The management of patients with IPF is multifaceted and consists of patient education and support, regular outpatient surveillance, symptom relief, pulmonary rehabilitation, annual vaccinations to prevent respiratory infection, identification and management of AE-IPF, supplemental oxygen, managing of comorbidities and ultimately palliative care or, in a minority of patients, referral for lung transplantation.
Two anti-fibrotic treatments became available for patients who meet stringent National Institute for Health and Care Excellence (NICE) criteria. Pirfenidone and Nintedanib neither cure, nor reverse the fibrosis, and have little impact on symptoms, but have been shown to reduce rates of lung function decline and, in the case of Pirfenidone, reduce AE-IPF and improve progression free survival.
Impact on health care systems: IPF is a cost- and resource-intensive disease encompassing hospitalisations, home-care and long-term-care, and anti-fibrotic therapy. The full health-burden on the NHS and UK economy is unknown but data from the British Lung Foundation, and projected estimates from our patient cohort, suggest that there are approximately 30,000 IPF diagnoses each year. Health-care costs alone for IPF are estimated at £11-57K per patient-year.
Need for biomarkers for precision management: Disease progression in IPF is highly variable with individuals experiencing very different trajectories. Response to anti-fibrotic therapy is also inconsistent with some patients tolerating the medication well and others experiencing significant side-effects. Currently, there is a lack of valid endpoints, apart from change in Forced vital capacity (FVC), which has poor sensitivity and specificity, to accurately assess disease activity or response to treatment. This makes it difficult to predict individual prognosis, or reliably detect early treatment response or failure which is important for developing treatment plans and providing patients with accurate prognostic information which allows them to plan for their future. Remote monitoring may allow clinicians and patients access to more granular longitudinal data on disease progression, rate of AE-IPF, and effects on QoL and begin to offer personalised treatment approaches in this cohort. Remote monitoring may also reduce patients’ attendance at hospital for clinical follow-up, or when taking part in clinical trials of novel agents. Remote monitoring may allow early identification of AE, and a better understanding of the frequency and impact of these events, and the potential to develop clinical trials of treatments in these patient groups.
1.3.2 Chronic Obstructive Pulmonary Disease (COPD)
COPD is a common, long term condition of the lungs that is usually caused by cigarette smoking. In addition to daily symptoms and limitations in activities, patients are prone to developing chest infections called ‘exacerbations’. Exacerbations are a significant problem: unpleasant for patients, and sometimes severe enough to cause hospital admission (and therefore NHS pressures) and death. Reducing the impact of exacerbations is very important. We have previously shown that earlier treatment of COPD exacerbations results in faster recovery, and reduced chance of hospital admission. Helping patients to detect exacerbations early is therefore important. We have also recently shown that monitoring heart rate and oxygen saturation via a finger probe may assist in this, especially overnight when the physiological signal is cleaner. Integration of these signals with additional symptom data, and use of innovative data analysis methodology, is likely to result in the greatest chance of supporting early detection of exacerbations, and assessment of disease progression. This is even more important in the era of COVID where many patients with COPD are classified as ‘clinically extremely vulnerable’ and thus remote monitoring provides the safest way to support management in partnership with their clinicians.
1.3.3 Post-Hospitalisation COVID19 Lung Disorders (PH-COVID)
Recovery from COVID19 has many unknowns, especially in the long term(2). Symptoms of COVID-19 have varied among those who have tested positive: some have displayed no symptoms, while others have developed severe pneumonia, progressing to lung injury and acute respiratory distress syndrome (ARDS) and, in the longer term, pulmonary fibrosis. Notably, the consequences of COVID-19 include effects on other organs including: heart, kidneys, and brain. Correspondingly, a diverse set of associations have been observed that together have been called ‘Long COVID’, a prolonged and delayed recovery from the acute illness including fatigue, shortness of breath and cough that is associated with mental health and neurological disorders such as fatigue, trauma and anxiety/depression(3,4). For those who were hospitalised, and have since been discharged, it is not yet clear what their medical, psychological and rehabilitation needs will be to enable them to make as full a recovery as possible.
Given this need to follow-up post-hospitalised COVID-19 patients, we consider remote monitoring to provide some key opportunities. Firstly, observation of chronic symptoms will necessitate home based monitoring as the scope of regularly interfacing with participants in-clinic may be limited due to the likelihood of further periods of lockdown and self-isolation of this population. Secondly, there needs to be a greater focus on understanding how daily life is affected by this disease. Remote monitoring therefore provides an ideal opportunity to collect multiple, continuous data streams from participants to report on physiology, QoL, environment and functional level. Building on our existing experience in using wearables to monitor participants who develop COVID-19(5), we aim to extend this to enable detailed observation of patients as they experience symptoms of Long-COVID. By taking a longitudinal high frequency and largely passive monitoring approach we aim to develop an understanding of disease trajectory and fluctuation of symptoms.